Lose 25+ pounds in Just 12 Weeks with Our Medical Weight Loss Program. Get a prescription in under 30 minutes.

AsherMed tailors the right weight loss medications and support so you can lose the weight, keep it off, and one day live med free.
SlimSmart powered by AsherMed Is more than just Medication
GLP-1 Medication Shipped to Your Door in 48-72 hours. *if prescribed
Full Patient Care with 1-1 Coaching with a Registered Nurse and Coach
Custom Nutrition Plans
Habit Based Changes NO Diets
Exercise programs tailored for YOU.
Medication management and side effect support.
Clinically proven and trusted weight loss programs.


How SlimSmart powered by AsherMed Works
We make medical weight loss seamless in 3 Easy Steps.
Take a brief survey
Input your Medical History
Pick Your Package
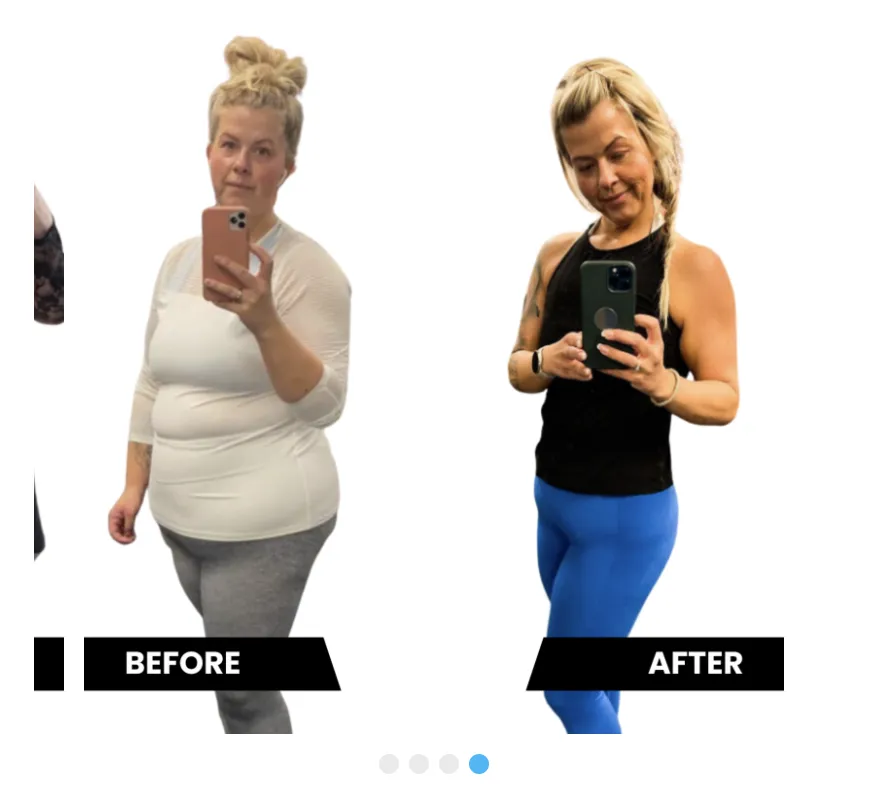
Will You Be The Next Success Story
Frequently Asked Questions
What Is It?
Compounded semaglutide, or GLP-1, is a prescription medication injected weekly to help people lose weight.Asher Med offers the base form of compounded semaglutide--not the sodium or acetate versions.
How Does It Work?
Compounded semaglutide mimics and helps amplify a hormone that naturally occurs in the body, called GLP-1.This hormone helps decrease blood sugar and slows down how quickly the stomach empties, so you feel fuller sooner and for longer.
Is It Safe?
Compounded semaglutide is commonly prescribed by weight loss specialists. It contains the same active ingredient as Ozempic® and Wegovy®Asher Med partners with licensed compounding pharmacies that are regulated by the FDA.
What's Included?
*INITIAL DOCTOR CONSULTATION
*1:1 PROVIDER CARE DEDICATED NURSE WEIGHT LOSS SPECIALIST
*FUNCTIONAL NUTRITION & HEALTH COACHING
*WEEKLY ACCOUNTABILITY AND CHECK UPS
*ASHER HABIT'S MATTER PROGRAM
*ASHER WORKOUT PROGRAMS
*MEDICATION
How To Take The Medication?
Compounded semaglutide is injected once a week. If prescribed, you’ll get detailed usage instructions in your treatment plan and video tutorials in the app.
Potential Side Effects?
Just like any medication, there’s always a potential for side effects. The most common side effects are constipation, nausea, and heartburn. These side effects are often managed with over-the-counter treatments.If you experience worrying symptoms or want to talk with a medical provider, you can message your Care Team any time through the Hims app. They’re here to help.
Important Safety Information
WARNING: RISK OF THYROID C-CELL TUMORS
See full prescribing information for complete boxed warning.
In rodents, SEMAGLUTIDE causes thyroid C-cell tumors in clinically relevant exposures. It is unknown whether SEMAGLUTIDE causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of SEMAGLUTIDE-induced rodent thyroid C-cell tumors has not been determined
SEMAGLUTIDE is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC and symptoms of thyroid tumors.
Do not take COMPOUNDED SEMAGLUTIDE if you:
Have a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN2).
Have been diagnosed with Diabetes (Type 1 or 2)Have been diagnosed with pancreatitis or history of pancreatitisHave severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food
Have a known allergy to semaglutide/any other GLP-1 drug or any of the inactive ingredients in COMPOUNDED SEMAGLUTIDE.
Inactive ingredients include: di-sodium hydrogen phosphate dihydrate, sodium chloride, benzyl alcohol, hydrochloric acid, sodium hydroxide pellets and water.
Have a history of suicidal attempts or active suicidal ideation
WARNINGS AND PRECAUTIONS
Acute Pancreatitis: Has occurred in clinical trials. Discontinue promptly if pancreatitis is suspected. Do not restart if pancreatitis is confirmed.
Acute Gallbladder Disease: Has occurred in clinical trials. If cholelithiasis is suspected, gallbladder studies and clinical follow-up are indicated.
Hypoglycemia: Concomitant use with an insulin secretagogue or insulin may increase the risk of hypoglycemia, including severe hypoglycemia.
Reducing the dose of insulin secretagogue or insulin may be necessary. Inform all patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
Acute Kidney Injury: Has occurred. Monitor renal function when initiating or escalating doses of COMPOUNDED SEMAGLUTIDE in patients reporting severe adverse gastrointestinal reactions or in those with renal impairment reporting severe adverse gastrointestinal reactions.
Hypersensitivity Reactions: Anaphylactic reactions and angioedema have been reported postmarketing. Discontinue COMPOUNDED SEMAGLUTIDE if suspected and promptly seek medical advice.
Females and males of reproductive potential: Discontinue COMPOUNDED SEMAGLUTIDE at least 2 months before a planned pregnancy because of the long half-life of COMPOUNDED SEMAGLUTIDE.
Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue COMPOUNDED SEMAGLUTIDE immediately
Diabetic Retinopathy Complications in Patients with Type 2 Diabetes: Has been reported in trials with COMPOUNDED SEMAGLUTIDE. Patients with a history of diabetic retinopathy should be monitored.
Heart Rate Increase: Monitor heart rate at regular intervals.
Suicidal Behavior and Ideation: Monitor for depression or suicidal thoughts. Discontinue COMPOUNDED SEMAGLUTIDE if symptoms develop.
Side EffectsMost common side effects (incidence ≥ 5%) in adults or pediatric patients aged 12 years and older are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distension, eructation, hypoglycemia in patients with type 2 diabetes, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis. To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
COMPOUNDED SEMAGLUTIDE delays gastric emptying. May impact absorption of concomitantly administered oral medications. Use with caution.
USE IN SPECIFIC POPULATIONS
Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue COMPOUNDED SEMAGLUTIDE.
Females and Males of Reproductive Potential: Discontinue COMPOUNDED SEMAGLUTIDE at least 2 months before a planned pregnancy because of the long half-life of COMPOUNDED SEMAGLUTIDE.
© 2024 Salsulsk LLC | Privacy Policy | Terms Of Service Address: 3325 S University Dr. Suite 108 Davie, Fl 33328 Phone: 754-202-4451